Growing global energy requirement are pushing the limits of solar technology. Scientists in Sweden have now taken a major step in the direction of unlocking the capability of halide perovskites.
Global requirement for electricity is climbing at a fast pace, making it important to find sustainable ways to fulfill future demands. One possible solution lies within the development of advanced solar cell materials which are far more efficient than the ones used today. These new materials could be produced so thin and flexible that they may cover everything from smartphones to complete buildings.
Researchers at Chalmers University of Technology in Sweden have lately made development in handling one of the most promising but difficult options: halide perovskites. By connecting computer-based simulations with machine learning, they’re starting to undo of the complex behavior of these materials.
According to the International Energy Agency, electricity already accounts for 20% of global energy use. Within the next 25 years, that share is anticipated to increase about 50%, in further emphasizing the importance of generating cleanser and more efficient energy technology.
“To meet the demand, there is a significant and developing requirements for new, environmentally friendly and efficient energy conversion methods, such as more efficient solar cells. Our findings are crucial to engineer and manage one of the most promising solar cell materials for most optimal use. It’s very interesting that we have simulation techniques which can answer questions that have been unresolved just a few years ago,” stated Julia Wiktor, the study’s principal investigator and an associate professor at Chalmers.
Promising materials for efficient solar cells
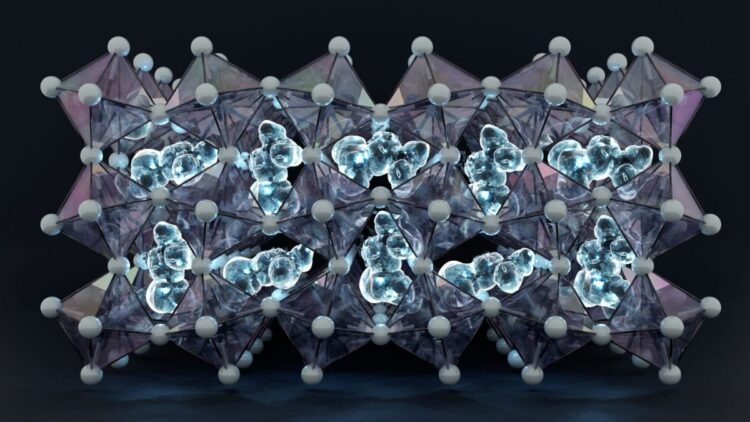
Materials lying within a group known as halide perovskites though about the most promising for generating cost-effective, pliable, and lightweight sun cells and optoelectronic devices which includes LED bulbs, as they integrate and release light extremely successfully. On the other hand, perovskite materials can decline rapidly, and knowing how best to use them needs a deeper understanding of why this happens and how the materials work.
Scientists have long struggled to understand one precise material in the group, a crystalline compound known as formamidinium lead iodide. It has excellent optoelectronic properties. Greater use of the material has been limited by its uncertainly however this will be solved via mixing two sorts of halide perovskites. However, more knowledge is needed about the two types so that researchers can best control the mixture.
The key to material design and control
A research group at Chalmers can now give a depth account of an crucial phrase of the material that has formerly been hard to give an explanation for by experiments alone. Understanding this phase is fundamental to being able of design and control both this material and mixtures based on it. The study was currently posted within the Journal of the American Chemical Society.
“The low-temperature phase of this material has long been a lacking piece of the research puzzle, and we’ve now settled a essential query about the structure of this section,” stated Chalmers researcher Sangita Dutta.
Machine learning contributed to the breakthrough
The researchers’ expertise lies in building exact models of various materials in computer simulations. This permits them to test the materials by exposing them to different scenarios and these are confirmed experimentally.
Nevertheless, modeling materials in the halide perovskite own family is tough, as capturing and deciphering their residences demand powerful supercomputers and lengthy simulation times.
“By combining our standard methods with machine learning, we’re now capable of run simulations that are thousands of times longer than before. And our models can now contain millions of atoms instead of hundreds, which brings them closer to the real world,” stated Dutta.
Lab observations match the simulations
The researchers identified the structure of formamidinium lead iodide at low temperatures. They could also see that the formamidinium molecules get stuck in a semi-stable state at the same time as the material cools. To make sure that their study models reflect reality, they collaborated with experimental researchers at the University of Birmingham. They cooled the material to – 200°C to make certain their experiments matched the simulations.
“We desire the insights we’ve received from the simulations can contribute to a way to model and analyze complicated halide perovskite materials in the future,” stated Erik Fransson, at the Department of Physics at Chalmers.